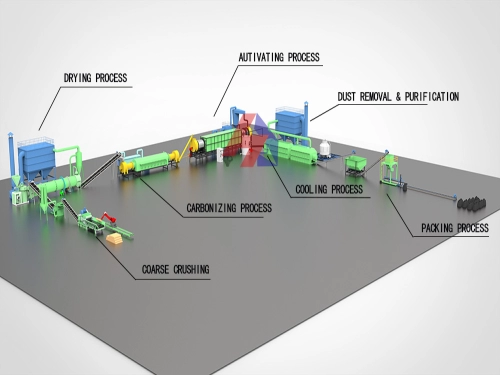
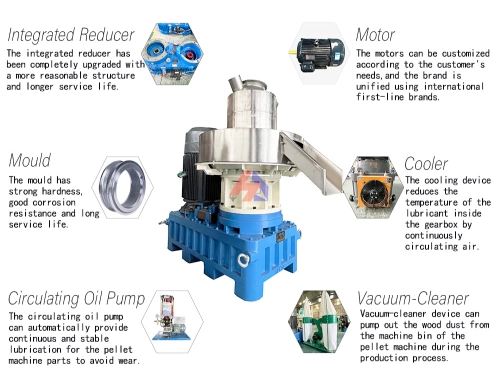
Why Hardwood Activated Carbon Dominates in Water Treatment
In critical water treatment applications—from municipal drinking water purification to the treatment of low-concentration organic wastewater—hardwood activated carbon has become the preferred choice. This preference stems from its unique combination of pore structure, adsorption properties, and physical characteristics, which align perfectly with the industry's core needs for efficiency, safety, and cost-effectiveness in large-scale, continuous operations.
Technical Superiority: A Perfect Match for Water Contaminants
The primary goal in water treatment is the removal of residual chlorine, small organic molecules, humic acids, odors, and trace colloids. Hardwood activated carbon excels here due to its optimal pore structure. Its porosity is dominated by a balanced network of mesopores (2-50 nm) and micropores (<2 nm). The micropores provide a vast surface area for capturing small molecules like chlorine and volatile organic compounds, while the mesopores act as conduits, efficiently adsorbing larger molecules like humic acids and preventing pore blockage to ensure long-term efficacy.
Furthermore, the surface chemistry of hardwood carbon, rich in oxygen-containing functional groups, gives it a natural affinity for polar pollutants common in water, such as chlorine and phenols, leading to 30-50% higher adsorption efficiency for these substances compared to some coal-based carbons. Its exceptional physical and chemical stability—exhibiting very low ash content, minimal leachates, and no heavy metal release—makes it safe for potable water applications and resilient across a wide pH range in industrial settings.
Practical Advantages for Real-World Operations
Beyond pure performance, hardwood activated carbon offers decisive benefits for system design and economics. Its balanced mechanical strength (≥90%) is high enough to withstand the physical stress of backwashing in filter systems, allowing for effective regeneration and reuse over multiple cycles. This significantly reduces long-term operational costs compared to carbons that degrade faster.
Its uniform particle size and moderate bulk density (0.4-0.5 g/cm³) enable even packing in filter vessels. This creates consistent flow paths with low pressure drop, preventing channeling and ensuring reliable treatment outcomes without frequent maintenance interruptions.
Finally, its superior cost-performance ratio seals its leading status. Produced from widely available raw materials like apricot or birch wood, hardwood carbon is far more cost-stable and affordable than imported coconut shell carbon. While coal-based carbon may have a lower initial price, its tendency to break down and need frequent replacement makes hardwood carbon the more economical solution over the lifecycle of a large-scale treatment plant.
Selection Guide: The Right Carbon for the Right Job
While hardwood carbon is the versatile champion for general water treatment, specific scenarios call for alternatives:
Hardwood Activated Carbon: The optimal all-rounder for municipal water, drinking water purification, and standard wastewater, where removing chlorine, odors, and organic matter cost-effectively is key.
Coconut Shell Carbon: Best for ultra-pure water production (e.g., electronics, pharmaceuticals) due to its exceptionally high microporosity for capturing trace organics.
Coal-Based Carbon: Suitable for specialized industrial wastewaters with high concentrations of large-molecule pollutants like dyes, where its macroporous structure is advantageous.
Summary
In summary, the dominance of hardwood activated carbon in water treatment is no accident. It represents the optimal intersection of targeted adsorption science, operational robustness, and practical economics, making it the most reliable and efficient workhorse for ensuring clean water on a large scale.
Copyright: Copyright belongs to Hengju Machinery! Reprint please indicate the source: https://www.hengjumachinery.com/industry-news/why-hardwood-activated-carbon-dominates-in-water-treatment.html